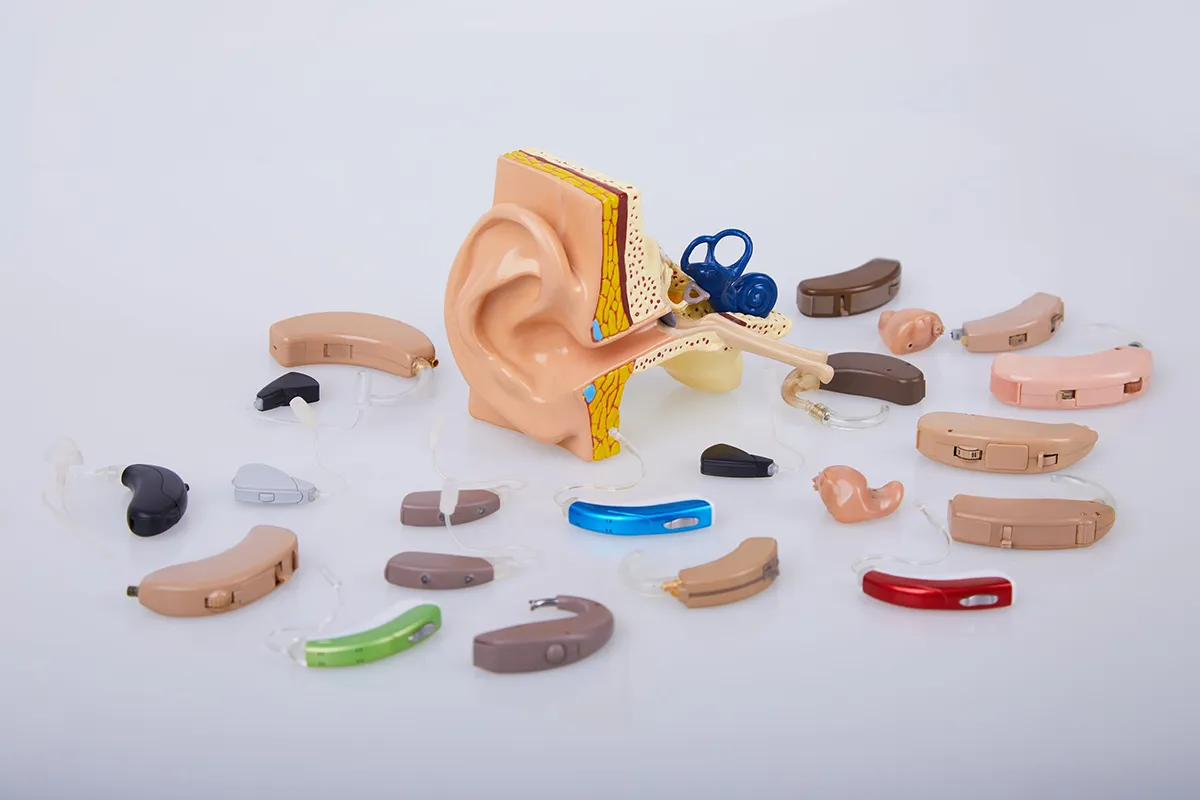
While wearing hearing aids can’t restore normal hearing, it could improve a person’s ability to hear speech in person or over phone calls, differentiate between a speaker and background noise, and generally improve one’s auditory experiences across different listening environments. In terms of how you acquire your hearing aids, there are two major categories: prescription hearing aids and over-the-counter (OTC) hearing aids, the second category having been newly designated and regulated by the U.S. Food & Drug Administration (FDA) in 2022.
Here, a representative from the Academy of Doctors of Audiology (ADA), a nationwide organization focused on promoting quality audiologic care, speaks to the similarities and differences between the two as well as the FDA requirements around OTC hearing technology.
OTC Hearing Aids vs. Prescription Hearing Aids: Similarities
“Over-the-counter hearing aids and prescription hearing aids have far more in common than they do differences,” says Dr. Victor Bray, M.Sc., Ph.D., FNAP, and a past president of the ADA. “They’re both medical devices. They’re both registered and cleared for distribution by the FDA. They have the same basic function: help people with hearing loss.”
Bray continues, “[OTC hearing aids are] not that much different than prescription hearing aids. They have microphones, they have amplifiers. They have the loudspeakers we call receivers. So the parts are the same, whether they’re in the prescription hearing aid category or the over-the-counter hearing aid. In many cases, these parts are made by the same companies. The devices are even assembled by the same companies that have been making traditional hearing aids that we’ve used for decades.”
When it comes to the similarities between OTC hearing aids and prescription hearing aids, both are forms of hearing technology that are considered medical devices and are regulated by the FDA. Both types amplify sounds for the wearers with the goal being to enhance their day-to-day auditory experiences, such as during phone calls, when trying to hear speech over background noise, and in other different listening environments.
OTC Hearing Aids vs. Prescription Hearing Aids: Differences
An OTC hearing aid or a prescription hearing aid can be worn by an adult aged 18 or older; however, the FDA states that, for children under the age of 18, prescription hearing aids are the only hearing aid option. And while both types of hearing aids help people with untreated hearing loss, OTC hearing aids are intended only for those with perceived mild to moderate hearing loss. For people with more severe hearing loss or profound hearing loss, prescription hearing aids are the only option.
Other differences between OTC hearing aids and prescription hearing aids revolve around accessibility. Prescription hearing aids require just that—a prescription from a licensed health care provider after you’ve undergone a medical exam and hearing test. Depending on where you live, you might also be required to buy your prescription hearing aid directly from a licensed point of sale.
Alternatively, a person doesn’t have to first undergo a medical exam or to acquire a prescription to buy OTC hearing aids, although some people might still choose to discuss their hearing health with their audiologist or ENT doctor beforehand. You can buy an OTC hearing aid directly from a brand, manufacturer, or retailer since there’s no requirement dictating a licensed point of sale. The FDA does recommend consulting a medical professional for more severe cases of hearing loss or for any “red flag” conditions, such as pain or discomfort in the ear, heavy earwax buildup, or sudden changes in hearing. Check the hearing aid product labeling or the FDA website for more information on when to seek medical advice.
Another major difference between non-prescription hearing aids and prescription hearing aids has to do with the fitting process. With prescription hearing aids, which can only be fitted by audiologists or other licensed hearing professionals, the doctor helps you assess not only the product’s physical fit but also how well the device’s programming is working for you. They can try to make adjustments based on your feedback.
In contrast to professionally fit hearing aids, some OTC devices are “self-fitting,” which means you have the ability to make adjustments to the device settings or programming from the comfort of your own home if you wish, such as through the use of a mobile app. In some cases, you might also have the ability to make adjustments to the physical fit of the new hearing aids, such as with a brand whose products account for differently sized ear canals.
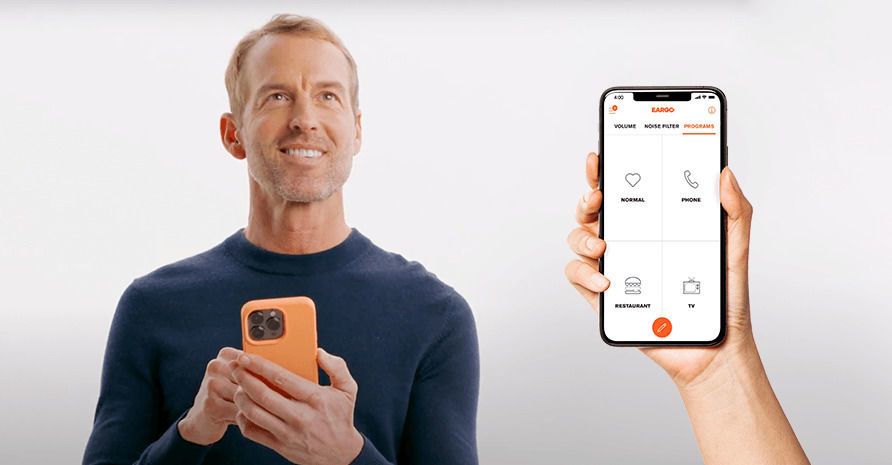 Some OTC hearing aids enable you to adjust certain settings through the use of the product’s mobile app.
Some OTC hearing aids enable you to adjust certain settings through the use of the product’s mobile app.
OTC Hearing Aids: FDA Requirements
The FDA regulates OTC hearing devices to ensure their safety and effectiveness for the wearer. “The FDA actually invoked a series of test requirements for quality control that OTC hearing aids have to meet to give assurance to the OTC hearing aid user that the products actually have quality,” Bray explains. “And it’s not even known whether or not some of those hearing aids that are being sold today as prescription hearing aids would pass the test requirements for the over-the-counter hearing aids. They’ve never been subjected to the same level of scrutiny that the FDA has put on over-the-counter hearing aids.”
The FDA’s requirements around over-the-counter devices include how the devices can be sold and purchased, what should and should not appear on the labeling inside and outside the boxes in the packaging, and how loud the devices can be. The FDA also requires an OTC hearing device to be adjustable by the wearer in terms of both frequency control and volume control. For self-fitting hearing aids, the wearer can personalize the sound quality of new OTC hearing aids even further by adjusting program selections or other digital features.
Are OTC Hearing Aids the Same as PSAPs?
OTC hearing aids and PSAPs (personal sound amplification products) do not refer to the same type of device. PSAPs simply amplify sound and are not designed with people with hearing loss in mind, and, therefore, it is not possible to adjust their frequency. Also, the FDA does not regulate PSAPs.
Is an OTC Hearing Aid or a Prescription Hearing Aid Better?
The ADA encourages people to find the right hearing aid for them. “From our standpoint, it doesn't matter whether the device itself is an OTC product or a prescription product,” says ADA Executive Director Stephanie Czuhajewski. “They all have the same components. It’s more about how the patient or the consumer is able to utilize that product to most effectively address their hearing loss or to optimize their hearing.”
Factors consumers can consider when reviewing their options include their personal degree of hearing loss, the product’s available sound modes and features (such as noise reduction), the product’s battery life (if they’re rechargeable hearing aids), the accessibility of the brand’s customer service department, and the availability and length of any kind of trial period. Additionally, some consumers might have a preference between behind-the-ear designs and completely-in-the-canal or CIC hearing aids. Ultimately, you should feel confident and comfortable when wearing hearing aids, so choose the pair that’s right for your needs.
Learn about more hearing health topics in this series by Eargo in collaboration with the ADA.
The information contained in multimedia content, interviews, or quotations from third parties (the “Content”) posted represents the views and opinions of the interviewed participants and does not necessarily represent the views or opinions of Eargo, Inc. (“Eargo”).
The production of the Content was paid for by Eargo, although the interviewed participants did not receive any money from Eargo for their participation in the creation of the Content. The Content has been made available for informational and educational purposes only and does not purport to be complete; nor is it intended to be a substitute for professional medical advice. Eargo does not make any representations or warranties with respect to the accuracy, applicability, fitness, or completeness of the Content.